Effects of Infoceuticals on Cellular Physiology: Towards a New Paradigm
Jacqueline A. Bonds1,2*, Ramamurthy Chitteti1,2*, Elena L. Kopp1,2*, and Juan P. Zuniga-Hertz1,2*
1Department of Anesthesiology, University of California, San Diego, La Jolla, United States
2Veterans Affairs San Diego Healthcare System, La Jolla, United States
*Authors contributed equally
Abstract
Living organisms, from the simplest organization to the more complex, all share the requirement of energy in order to perform basic functions that sustain life and ensure its continuity. Cellular physiology requires complex mechanisms to maintain homeostasis that are activated when normal parameters change. Ancient medical practices like acupuncture suggest that many of the human health impairments occur because of an energetic imbalance. In this regard, NES Health has developed the concept of "Infoceuticals" -- remedies designed to restore the body's energetic balance, or the means by which it is powered and communicates wirelessly. These remedies may be administered through a special preparation of mineralized water ("imprinted" with photons and magnetic fields), or imprinted directly into the body via electronic equipment. In this report, we used a multicellular platform to determine the effect on cellular physiology of diverse Infoceuticals both in liquid form and through direct imprinting. We observed unique and cell-specific effects in terms of cellular membrane structure, mitochondrial function, oxidative stress, and viral infection protection. These findings aim to provide basic science insights to the process of understanding the underlying phenomena that take place after Infoceutical treatments.
Keywords: Infoceuticals, cell physiology, metabolism.
Introduction
Life, from its simplest forms to higher organisms, presents common factors or functions like growth, reproduction, response to stimuli, metabolism, beside others. All these functions present another common element: all require energy to happen. Since energy cannot be created on a thermodynamic system, it is conserved through them (first law of thermodynamics) and is transformed from one form to another. Energy flows in living systems in manners which allow for its conversion and transformation for use in many processes. Such is the case for electrochemical gradients and the consequent electromotive force that leads to synthesis of biomolecules, storage of energy in chemical structures and bonds, movement, cellular communication, and virtually all biological processes.
The same way potential energy is “stored” in a dam, the generation of gradients throughout the cellular membranes is used to accumulate potential energy, giving to the cellular membranes a cornerstone role for life. For this reason, membranes can be referred to as energetic capacitors [1]. During the geological eras, membranes evolved into complex systems which based on their biochemical composition, with greater emphasis in their lipids and proteins, have permitted complex forms of life and all the underlying bioenergetic mechanisms to exist [2]. However, it is critical to understand that energy can assume diverse forms, ranging from potential energy, chemical energy, electricity and many others.
Holistic medicine practices and techniques, such as acupuncture, suggest the existence of energetic fields [3, 4]. NES Health suggests the existence of Infoceuticals that can affect these energetic fields. These Infoceuticals are water formulations which have been subjected to specific electromagnetic frequencies, each carrying unique information that can differentially effect living cells in vitro [5]. Alternatively, these Infoceuticals can be introduced to living cells via direct technology imprint of the same information.
In order to obtain quantifiable results on how various Infoceuticals impact cellular physiology, we performed a battery of in vitro experiments using various types of cells: cardiac muscle, skeletal muscle, neural stem cells, and lung epithelial cells. This study investigates potential effects on biophysical parameters such as membrane rigidity and fluidity, mitochondrial proliferation, cellular stress, and the potential to provide protection against viral infections. The aim of the study is to determine the extent to which various Infoceuticals modulate general aspects of the cell physiology and behavior in vitro.
Methods
Cell culture
C2C12 (CRL-1772™), H9C2 (CRL-1446™), and A549 (CRM-CCL-185) cell lines were purchased from American Type Culture Collection (ATCC, Manassas, VA, USA). C2C12 cells (mouse myoblast) and H9C2 cells (cardiomyoblasts from embryonic rat hearts) were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) (GIBCO/BRL, 11885092) containing 5mM glucose, supplemented with 10% Fetal Bovine Serum (FBS, Thermo Fisher Scientific, 16000044) and 1% Penstrep (Penicillin/Streptomycin, GIBCO, 15140122). All used culture media were sterile filtered with EMD Millipore Stericup Sterile Vacuum Filter Units (Fisher Scientific, SCGPU05RE). The cells were maintained in vented 75cm2 tissue culture flasks (Sarstedt, Germany) and incubated at 37°C under an atmosphere of 95% air and 5% CO2. The cultures were routinely split, using trypsin (0.25% Trypsin-EDTA, Gibco, 25200-056) at 50-60% confluency. To keep the cultures in low passages, stocks were frozen in liquid nitrogen at passage one to three (DMEM with 5% DMSO). To preserve the characteristics of the cell lines, treatments of C2C12 and H9C2 myoblasts were started in passage 2-8 in all experiments.
Primary Neurospheres
Culture. Primary neurospheres (neural stem cells) were thawed from multiple isolations (neurospheres were isolated from adult mice as previously described [6]). In brief, each vial of neural stem cells was rapidly thawed in a 37°C water bath. Cells were washed in 9mL of warm growth media (DMEM/F-12 [Thermo Fisher Scientific 11320082], 1% penicillin-streptomycin, 20% B27 supplement [Gibco, 17504044], 10% N2 supplement [Gibco, 17502001]) and centrifuged at 1000rpm for 5 minutes at RT. Cells were resuspended in growth media EGF, bFGF, and noggin. Neurospheres were cultured for 7 days with growth factors (EGF, bFGF, and noggin) added every 48-72 hours.
Passaging. Media containing neurospheres was collected and spun at 1000 rpm for 5 minutes at RT. Neurospheres were dissociated by resuspending in 1.25 mL of 0.05% trypsin-EDTA and incubated in a 37°C water bath for 7 minutes. 3 mL of Trypsin inhibitor was added, and cells spun at 1000 rpm for 5 minutes. Cells were then counted (BioRad Automated Cell Counter) and plated at 5,000 cells/cm2 for experiments.
Infoceuticals treatment
H9C2, C2C12, and A549 were treated during 72h; cell culture media was replaced daily to add the Infoceutical at specific dilution. For neurospheres, the Infoceutical was added directly to the media every 24 hours. For membrane and cellular oxidative stress studies, H9C2 cells were treated with ED3 Cell Driver (CD) (dilutions 1:100, 1:1,000, 1:10,000); for cellular metabolism, H9C2 and C2C12 cells were treated with ED3 Cell Driver. For viral protection via Energetic Terrains, A549 cells were treated with the respective Infoceuticals for 1 hour prior to 24 hours of viral exposure. In addition, we used the NES Health imprinting device in order to treat H9C2 and A549 cells to determine the effect of ED13 imprinting frequencies in viral infection protection.
Electron paramagnetic resonance spectroscopy membrane fluidity analysis
After Cell Driver (ED3) treatment, H9C2 cells membrane fluidity was determined by electron paramagnetic resonance spectroscopy (EPR) as previously described [2]. Briefly, cells were resuspended after 5min incubation with trypsin 0.25% at 37ºC and subsequently washed in ice cold PBS 1X. Cells were resuspended in 200mL of complete culture media, split in two 100mL aliquots and incubated with spin probes 5 and 16-Doxyl stearic acid (5-DSA, 16-DSA), respectively. Cells were incubated 5min on ice and 5min at 25 ºC. Un-bound spin probe was removed by subsequent wash and resuspension in fresh complete media. Samples were placed in 50mL Magnetech capillaries and analyzed in a Magnetech MiniScope MS400 benchtop spectrometer. EPR conditions include microwave power of 7mW, modulation amplitude of 2G; modulation frequency of 100kHz, sweep with of 141.21G centered at 3357.90G, and a scan rate of 7.31G/s. The spectrum of 5-DSA analysis is used to determine membrane fluidity/rigidity parameters; rotation correlation time (t) of the spin probe was obtained from the 16-DSA spectrum analysis as previously described [7].
H9C2 Mitochondrial transmitted electron microscopy
6-well plates of confluent cells were fixed with 1% glutaraldehyde, 4% paraformaldehyde in 0.1M cacodylate buffer for 1 hour at room temperature and overnight at 4ºC. After washing, the cells were post-fixed with 1% OsO4 and en bloc stained with 2% uranyl acetate for one hour each. After dehydration through a standard ethanol series, cells were transitioned with hydroxypropyl methacrylate (HPMA; Ladd Scientific #21328) to LX-112 (#21310 Ladd Scientific) and embedded as monolayers. After polymerization at 60ºC, pieces of monolayers were mounted on blocks using superglue and dried overnight prior to sectioning. Sections of 70-75nm were stained with uranyl acetate and lead nitrate before viewing on Tecnai T-12. Mitochondria from all cell areas, plasma membrane associated, perinuclear and free cytosolic populations were randomly sampled. Mitochondrial count and area calculations were analyzed with ImageJ software.
BrdU Cell Proliferation Assay
Neurospheres were single cell dissociated using trypsin as described above. Cells were counted and plated at 5000 cells/well in a 96-well plate. This assay was performed using a commercially available kit from BioVision (BrdU Cell Proliferation Assay Kit, K306). In brief, 5-Bromo-2’-dioxyuridine (BrdU) was diluted to 1X in each well and cells cultured for 24 hours. Plates were centrifuged at 1000 rpm for 5 minutes and the manufacturers protocol was followed.
Information imprinting viral infection analysis
To test the effect of Infoceuticals preventing viral infection, A549 cells were plated in 96-well plates (5x105 cells/well) and treated with imprinter set at ED13 (Immunity Enhancer Infoceutical) for 90 seconds over the course of 72 hours. Subsequently, cells were treated with an engineered viral construct consisting of SARS-CoV-2 pseudovirus where the genetic material was replaced by red fluorescent protein (RFP) expression system; the cells were also treated with variant strains of the virus (Beta, Delta, UK, D614G, Lambda, and Brazil variants). When the viral construct (pseudovirus) infects a lung cell, the RFP is expressed in the cytosol and red fluorescence can be measured as a means of viral infection. Red fluorescence was measured after 24h of viral exposure in a Tecan Spark 10M fluorescence plate reader at 535-590nm. Red fluorescence was normalized by nuclear staining with DAPI. Values are expressed as RFP/DAPI. In addition, live immunofluorescence imaging of A549 cells was performed. The red fluorescence is an indicator of cells that were infected. DAPI was used as nuclear staining control.
Reactive Oxygen Species analysis
To evaluate the effect of the Cell Driver Infoceutical (ED3) on cellular oxidative stress due to reactive oxygen species (ROS), we cultured H9C2 cells on a glass-bottom 96-well plate (Celllvis-P96) and treated the cells (3.5x106cells) with Cell Driver for 72 hours. After this time, cells were incubated with CellROX green ROS detection reagent at final concertation of 1μM per well for 4 to 6 hrs at 37 ºC. After completion of incubation, the cells were washed with PBS 1X. The ROS mean signal intensity was quantified using Citation 5 (Bioteck) and images in software (Gen5).
Analysis
All results are expressed as mean ± SD and analyzed by one-way ANOVA variance test followed by Tukey’s test for multiple comparison. A p value <0.05 was considered as a statistically significant difference.
Results
Chronic Cell Driver Infoceutical (ED3) treatment induces cellular membrane biophysical changes
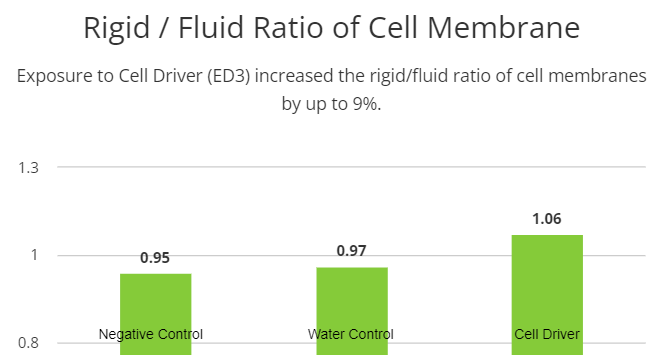
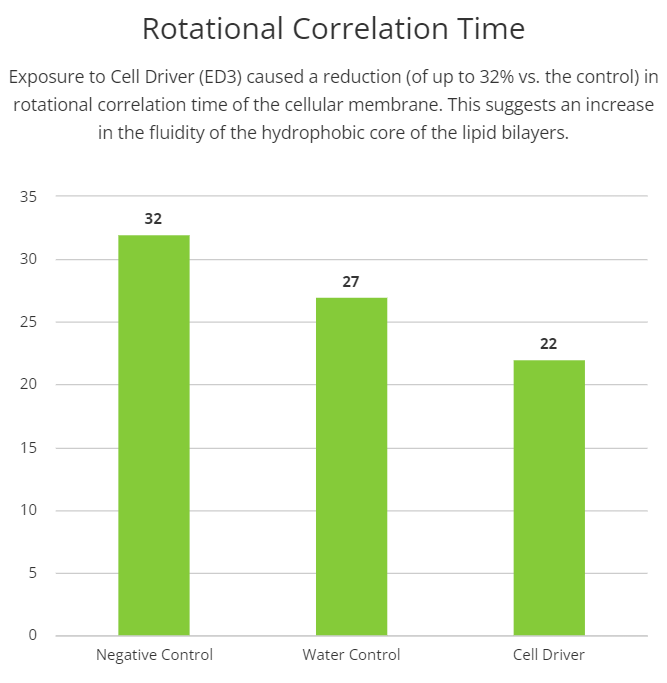
Cellular membranes are more complex than mere boundaries of the cell. They behave like organelles with complex and dynamic structural organization that controls the internal environment of the cell, substances exchange, communication, besides others. In order to evaluate possible effects of the Cell Driver Infoceutical in cellular membranes, we treated H9C2 cells with three different dilutions of Cell Driver and studied membrane biophysical parameters like fluidity and rigidity in different regions of the lipid bilayer (i.e., near the polar headgroups and the hydrophobic core) by electron paramagnetic resonance spectroscopy (EPR). To assess membrane fluidity and rigidity near the polar head groups of the lipid bilayers, we used 5-doxyl stearic acid. We observed an increase in the rigid/fluid ratio in the cells treated with 104 dilution of Cell Driver (1.06±0.035, n=5). Interestingly, by using 16-doxyl stearic acid, we observed a reduction in rotational correlation time, suggesting an increase the fluidity of the hydrophobic core of the lipid bilayers (22.47±3.24, n=5). These results suggest that chronic exposure to Cell Driver induced changes in the cellular membranes that can be associated with distinct cellular functions.
Visual Summary of Findings:
Chronic Cell Driver Infoceutical (ED3) treatment promotes mitochondrial proliferation in H9C2 cells
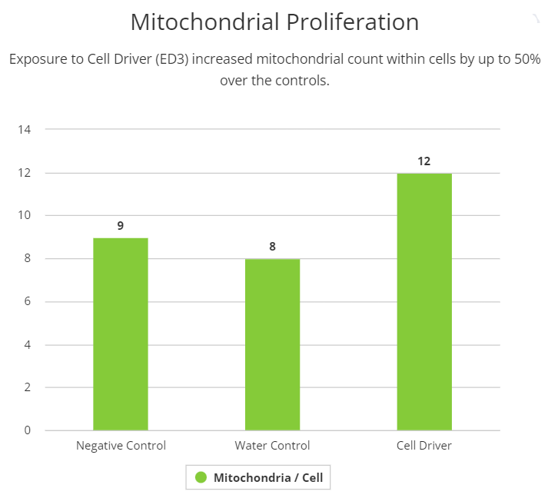
To investigate mitochondrial structural effects of Cell Driver Infoceutical, we studied mitochondrial morphology through transmitted electron microscopy. After 72h treatment with Cell Driver at three different dilutions, mitochondrial inspection in H9C2 cells showed significant difference in mitochondrial numbers per cell, without changes in mitochondrial area. These data suggests that Cell Driver may induce mitochondrial fusion and fission dynamics, increasing their number.
Visual Summary of Findings:
Chronic Cell Driver Infoceutical (ED3) treatment reduces cellular oxidative stress
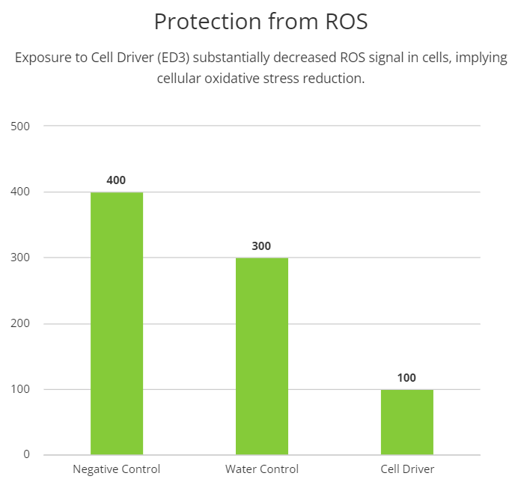
Elevated levels of reactive oxygen species (ROS) have been associated with cellular damage. Thus, we treated cells with Cell Driver Infoceutical followed by measures of cellular ROS by fluorescence of CellROX dye, which produces a strong green fluorescence signal in the presence of high levels of ROS. When H9C2 cells were treated with Cell Driver, a significant reduction in the ROS signal was observed, compared with the control (p=0.0079), suggesting that Cell Driver may induce a reduction in cellular oxidative stress.
Visual Summary of Findings:
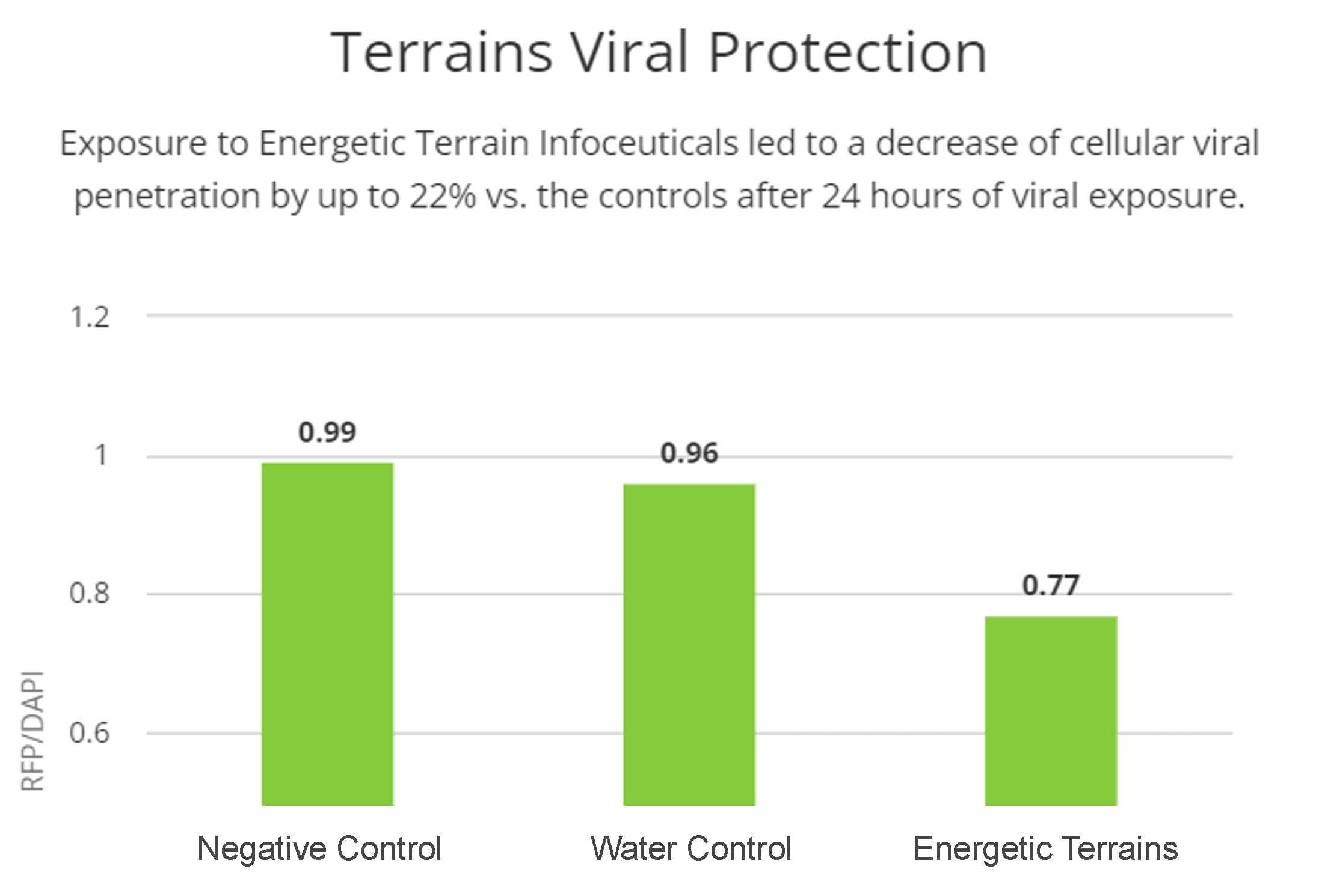
Energetic Terrain Infoceuticals protect lung epithelial cells from viral infection
Considering the structural changes observed, we decided to investigate the possible protective effect of the two Energetic Terrain Infoceuticals (ET2 and ET3) against two different pseudoviruses (SARS-CoV-2 and HIV respectively) and evaluated lung epithelial cells (A549) infection. We exposed cells for one hour to negative and water controls as well as the Infoceuticals, then exposed them for 24 hours and measured viral penetration in each set of cells. In both cases, cells exposed to the Infoceuticals showed far less viral penetration, suggesting a real viral protective effect of the Infoceuticals.
Visual Summary of Findings:
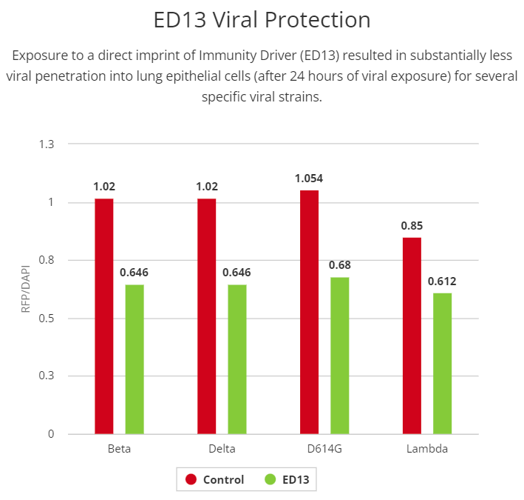
Direct Imprinting of Immunity Driver (ED13) promotes strain-specific viral protection
In this research, we used a direct technology imprint of the Infoceutical into cells, rather than using a liquid Infoceutical. To evaluate the potential that Immunity Driver imprinting might have in the host-pathogen interaction, we evaluated the potential to protect lung epithelial cells from viral infection. When A549 cells were treated with Immunity Driver prior to viral exposure, we observed a significant reduction in the RFP/DAPI ratio for many but not all variants of coronavirus tested. Protective effects were seen with the following strains: Beta (0.90±0.024, p<0.0001), Delta (0.89±0.027, p<0.0001), D614G (0.91±0.028, p<0.0001), and Lambda (0.90±0.036, p<0.0001) variants of coronaviruses. These results suggest that the Immunity Driver imprinting triggered a protective effect that is strain specific.
Visual Summary of Findings:
Discussion
In this study, the aim was to investigate the effects of various Infoceuticals on cellular physiology and behavior. We used several different Infoceuticals, each of which contains unique types of information. These Infoceuticals were prepared by NES Health parameters [5]. Cell Driver (ED3) is designed to promote cellular energy, metabolism, and communication, and is associated with various immune cells, including mast cells and immunoglobin E. Immunity Driver (ED13) is associated with precursors to all immune cells, mature cells of the innate immune system, and the spleen’s role in immunity. Energetic Terrain Infoceuticals are designed to promote the body's healing and protective functions especially through communication with DNA and RNA.
Cellular membranes can be considered as an organelle with complex functions and deep implications for cell physiology rather than simply housing organelles. In this regard, we studied membrane biophysical parameters after treating with Cell Driver in order to determine the impact on membrane structure, and consequently its function. We used 5-DSA, which is a probe commonly used to assess membrane rigidity and fluidity parameters near the polar head groups. Our data suggests that there is an increase in the rigidity/fluidity ratio when we use a high dilution of Cell Driver. The increase in this ratio is associated with a more organized membrane. In other words, when this ratio is higher, it is expected that freely moving proteins in the membrane are more organized and thus better able to carry out membrane specific functions.
Additionally, we measured the membrane fluidity in the hydrophobic core of the membrane by using 16-DSA. Interestingly, we observe an increase in the fluidity near the center of the membrane (smaller rotation correlation time). The center of the hydrophobic core of the membrane is fluid by nature, and it is known that nonpolar molecules can cross through the membrane. Thus, an increase in the fluidity of the hydrophobic core may facilitate the transport of nonpolar molecules such as oxygen [2]. The changes reported here are mostly structural, therefore, further experiments must be done in order to definitively conclude what the functional impact is due to these biophysical shifts.
Mitochondria have long been considered as the ‘powerhouse of the cell’ due the production of ATP which is the cell's energy currency. The generation of ATP is dependent on both the physical ultrastructure of the mitochondria, as well as its metabolic function. We first tested the Cell Driver Infoceutical in H9C2 cells and analyzed mitochondria ultrastructure by electron microscopy. We observed an elevation in the number of mitochondria per cell at a lower dilution of Cell Driver.
As further evidence of benefits associated with Cell Driver, we measured generation of ROS which is associated with cellular oxidative damage. ROS are common signaling molecules. However, when levels of ROS are outside of the normal range, these species can become toxic to the cell. Our data shows that the Cell Driver significantly reduced ROS production, which implies that there is a protective effect.
It is also of great interest to investigate the quantifiable effectiveness of a holistic approach to prevent viral infection. Here we investigated if Infoceutical treatment could confer cellular protection against infection of the SARS-CoV-2 virus. In this experiment we used a pseudovirus which induces RFP expression in cells that have taken up the virus. Excitingly, there is decreased expression of RFP in cells treated with the ET2 Infoceutical, indicating decreased viral infection of the COVID-pseudovirus. We saw similar results in terms of protection against an HIV pseudovirus and the ET3 Infoceutical. These results suggest that treatment with the Energetic Terrain Infoceuticals may confer protection against certain viral infection. Given these observed biological changes following Infoceutical treatment, which are beneficial to cellular health and immune response, it is critical for more extensive studies to be performed in order to fully understand the mechanism underlying them.
Lastly, we wanted to understand if the benefit observed using the Infoceutical waters could be replicated using an imprinter which could impart information directly onto the cells. We quantified the ability of the SARS-CoV-2 pseudovirus and its variants to infect lung epithelial cells following imprinter exposure. We observe significant decreases in RFP signal of certain COVID variants following imprinting. These results suggest that the imprinting is not only conferring some type of protection against viral infection, but also that it is doing so through a unique mechanism. Additional experiments are necessary to fully elucidate these observations.
In summary, this work presents evidence that both the Infoceuticals and imprinting may exert positive biological effects on cellular physiology. It is important to note that these effects are not equal between either the type of treatment (water versus imprinting) or cell types. Observations from this study are the first of its kind, and thus more comprehensive experiments are necessary to understand the mechanisms underlying these changes.
Acknowledgement
We acknowledge Ingrid Niesman electron microscopy images generation at the SDSU EM Facility in the Department of Biology.
Literature Cited
- Ray, S., et al., The plasma membrane as a capacitor for energy and metabolism. Am J Physiol Cell Physiol, 2016. 310(3): p. C181-92.
- Zuniga-Hertz, J.P. and H.H. Patel, The Evolution of Cholesterol-Rich Membrane in Oxygen Adaption: The Respiratory System as a Model. Front Physiol, 2019. 10: p. 1340.
- Van Hal M, D.A., Green MS. Acupuncture. [Updated 2021 Jul 31]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK532287/.
- Wang, T.H., et al., Comparison of physical electrical conductivity and acupuncture de-qi sensation between stainless steel needling and supercritical fluid-treated needling. Biomed J, 2021. 44(6 Suppl 2): p. S267-s274.
- Fraser, P.H., Energy and Information in Nature. 2012: Choice Point Communications. Peter H. Fraser.
- Massey, H., Restore Your Energy with Bioenergetics. 2019: NES Health®.
- Bonds, J.A., et al., Deficits in hippocampal neurogenesis in obesity-dependent and -independent type-2 diabetes mellitus mouse models. Sci Rep, 2020. 10(1): p. 16368.
- Ogura, R., et al., ESR spin-labeling method of determining membrane fluidity in biological materials--tissue culture cells, cardiac mitochondria, erythrocytes and epidermal cells. Kurume Med J, 1988. 35(4): p. 171-82.
- Yao, J., Y. Mu, and F.H. Gage, Neural stem cells: mechanisms and modeling. Protein Cell, 2012. 3(4): p. 251-61.
- Lazarov, O. and R.A. Marr, Neurogenesis and Alzheimer's disease: at the crossroads. Exp Neurol, 2010. 223(2): p. 267-81.
- Ivanova, A., et al., Synthetic Thymidine Analog Labeling without Misconceptions. Cells, 2022. 11(12).
- Demars, M., et al., Impaired neurogenesis is an early event in the etiology of familial Alzheimer's disease in transgenic mice. J Neurosci Res, 2010. 88(10): p. 2103-17.
- Gao, J., et al., In vitro investigation of the mechanism underlying the effect of ginsenoside on the proliferation and differentiation of neural stem cells subjected to oxygen-glucose deprivation/reperfusion. Int J Mol Med, 2018. 41(1): p. 353-363.
- Si, Z., et al., Heme Oxygenase 1 Inhibits Adult Neural Stem Cells Proliferation and Survival via Modulation of Wnt/beta-Catenin Signaling. J Alzheimers Dis, 2020. 76(2): p. 623-641.

